 EXOSOMES, SECRETOME, CANCER AND SYSTEMS BIOLOGY EXOSOMES, SECRETOME, CANCER AND SYSTEMS BIOLOGY
|
|
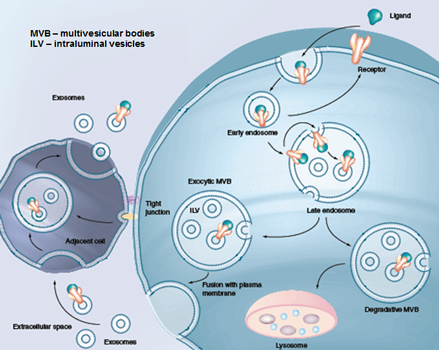
Our major research interests are in exploring the role of extracellular matrix components (soluble secreted proteins and extracellular vesicles) in cancer and intercellular communication.
Our lab integrates proteomic, genomic and bioinformatics methodologies to study cancer progression. In addition to medical research, our lab is also interested in basic science projects including the biogenesis of exosomes and
intercellular communication.
|
 EXOSOMES IN THE TUMOR MICROENVIRONMENT EXOSOMES IN THE TUMOR MICROENVIRONMENT
|
|
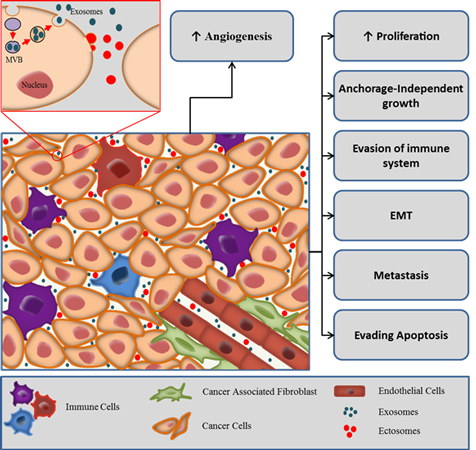
Exosomes are 40-100 nm diameter membrane enclosed extracellular vesicles released by various cell types, including cancer cells. For tumors to progress, bidirectional crosstalk
between different cells occurs within the tumor and its surrounding supporting tissue. A tumor can be considered as a complex tissue or organ with abnormal cells harbouring genetic mutations,
typically referred to as tumor or cancer cells, enmeshed within the surrounding and interwoven stroma, the epithelial parenchyma, which provides the connective tissue of the tumor.
Stromal elements include the extracellular matrix as well as other cell types that are activated and/or recruited to the tumor microenvironment such as fibroblasts, immune and inflammatory cells,
fat cells and endothelial cells of the blood and lymphatic circulation. Recent literature indicated that all aspects of cellular tumorigenicity are profoundly influenced by reciprocal
interactions between responding normal cells, their mediators, structural components of the extracellular matrix, and genetically altered neoplastic cells. Exosomes have recently been
recognized as important mediators of the cross-talk in the tumor microenvironment. Exosomes derived from tumor cells have been shown to have both pro- and anti-tumorigenic properties. Our lab is interested in studying
the role of exosomes in the tumor mivroenvironment.
Reference:
Gangoda, L., Boukouris, S., Liem, M., Kalra, H. and Mathivanan, S. (2015) Extracellular vesicles including exosomes
are mediators of signal transduction: Are they protective or pathogenic? Proteomics. 15:260-71. 
|
 PROTEOGENOMICS ANALYSIS OF EXOSOMES AND EXTRACELLULAR VESICLES PROTEOGENOMICS ANALYSIS OF EXOSOMES AND EXTRACELLULAR VESICLES
|
|
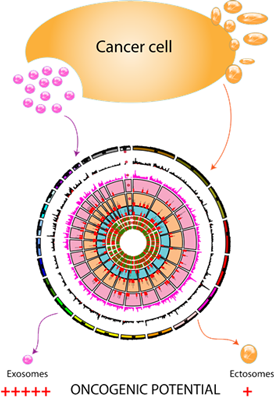
Recent studies have highlighted the secretion of oncoproteins including mutant proteins via exosomes. However, a prior knowledge of the mutant protein is a prerequisite in all of the
published studies. A global approach to systematically identify mutant proteins secreted through exosomes will aid in elucidating the functional roles of exosomes. In order to identify the mutant proteins that are secreted by a
cell via exosomes, we use global proteogenomics approach. In addition to functional implications, as exosomes may contain disease causing proteins including mutant proteins/RNA, assaying for mutant or disease causing proteins/RNA as disease biomarkers
may provide the required specificity for a biomarker test.
Reference:
Keerthikumar, S., Gangoda, L., Liem, M., Fonseka, P., Atukorala, I., Ozcitti, C., Mechler, A., Adda, C.G., Ang, C.S. and Mathivanan, S. (2015)
Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes.
Oncotarget. 17, 15375-15396. 
Boukoris, S. and Mathivanan, S. (2015) Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics clinical applications. 9, 358-67.

|
 SYSTEMS BIOLOGY - EXOSOMES AND COLORECTAL CANCER SYSTEMS BIOLOGY - EXOSOMES AND COLORECTAL CANCER
|
|
Constant dynamic interactions between a cell and its surrounding tissue microenvironment are important in maintaining the differentiated state of a cell.
While such organised intercellular signalling cascades are pivotal in cellular proliferation, sustained disruption of key signalling events render the cells susceptible to malignancy.
Our group uses systems biology or bioinformatics approaches to study the molecular mechanisms of colorectal cancer. We use proteomic and genomic technologies to study colorectal cancer cells
and integrate bioinformatics methods to make biological sense of the obtained data. With the explosion of datasets from high-throughput techniques, systems biology approaches hold immense
promise to investigate such data and present them at the context of the disease. It has to be noted that high-throughput data should be dealt carefully owing to the noise and systemic pitfalls.
Our group develops computational tools to analyze such datasets and integrate them with heterogeneous datasets obtained from similar biological experiments using statistics and computation.
Reference:
Pathan, M., Keerthikumar, S., Ang, C.S., Gangoda, L., Quek, C.M.J., Williamson, N.J., Mouradov, D., Sieber, O.M.,
Simpson, R.J., Salim, A., Bacic, A., Hill, A.F., Stroud, D.A., Ryan, M.T., Agbinya, J.A., Mariadasson, J.M., Burgess, A.W. and Mathivanan, S. (2015)
FunRich: a standalone tool for functional enrichment analysis. Proteomics. 15, 2597-2601.

|
 IDENTIFICATION OF DIAGNOSTIC AND PROGNOSTIC BIOMARKERS IDENTIFICATION OF DIAGNOSTIC AND PROGNOSTIC BIOMARKERS
|
|
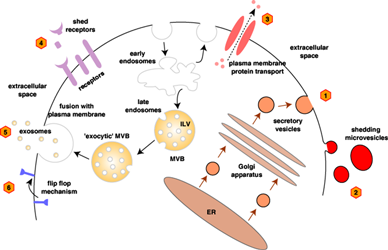
Secretome can be defined as the total set of proteins released from normal and/or cancer cells into the surrounding microenvironment.
In eukaryotic cells, proteins can be secreted by classical and non-classical secretory pathways.
It is estimated that about 10-15% of the proteins encoded by the human genome can be secreted by the well characterized classical secretory pathway.
The secretome reflects the functionality of the cancer cells and includes proteins such as growth factors, extracellular matrix-degrading enzymes, cell motility factors, angiogenic factors; ectodomain shed
receptors and immunoregulatory cytokines participating in various physiological processes such as immune defense and cell signaling. Such bioactive molecules play critical roles in pathological processes
including cancer cell differentiation, invasion, metastasis and sustained angiogenesis by regulating cell-cell adhesion and extracellular matrix interactions.
Our group explores colorectal cancer cell line secretome to identify secreted proteins that can be used as biomarkers for diagnostic and prognostic purposes.
|
 FUNDING FUNDING
|
|
|
Australian Research Council (ARC)
National Health and Medical Research Council (NHMRC)
National Institutes of Health (NIH) - USA
Ramaciotti Foundation
ANZ Trustees
La Trobe University
|